Healthtech
Innovation
My Experience Uncovering Innovation Opportunities in Developping Healthcare Markets.
From 2011 to 2017, I was Design Manager and Human Factor Engineer (HFE) consultant at Worrell's Shanghai branch, a global design firm for healthcare innovation and strategy.
I led a team of Industrial and UX designers working in close collaboration with our Design Research and Engineering teams.
Using human centered and design thinking methodologies, we supported Global and local Healthcare Brands like Jonhson & Johnsons, Baxter, Neusoft and Medtronics in identifying innovative product and services opportunities for the Chinese market. Taking these potentials all the way from a field insights to concepts, pilots and commercial products.
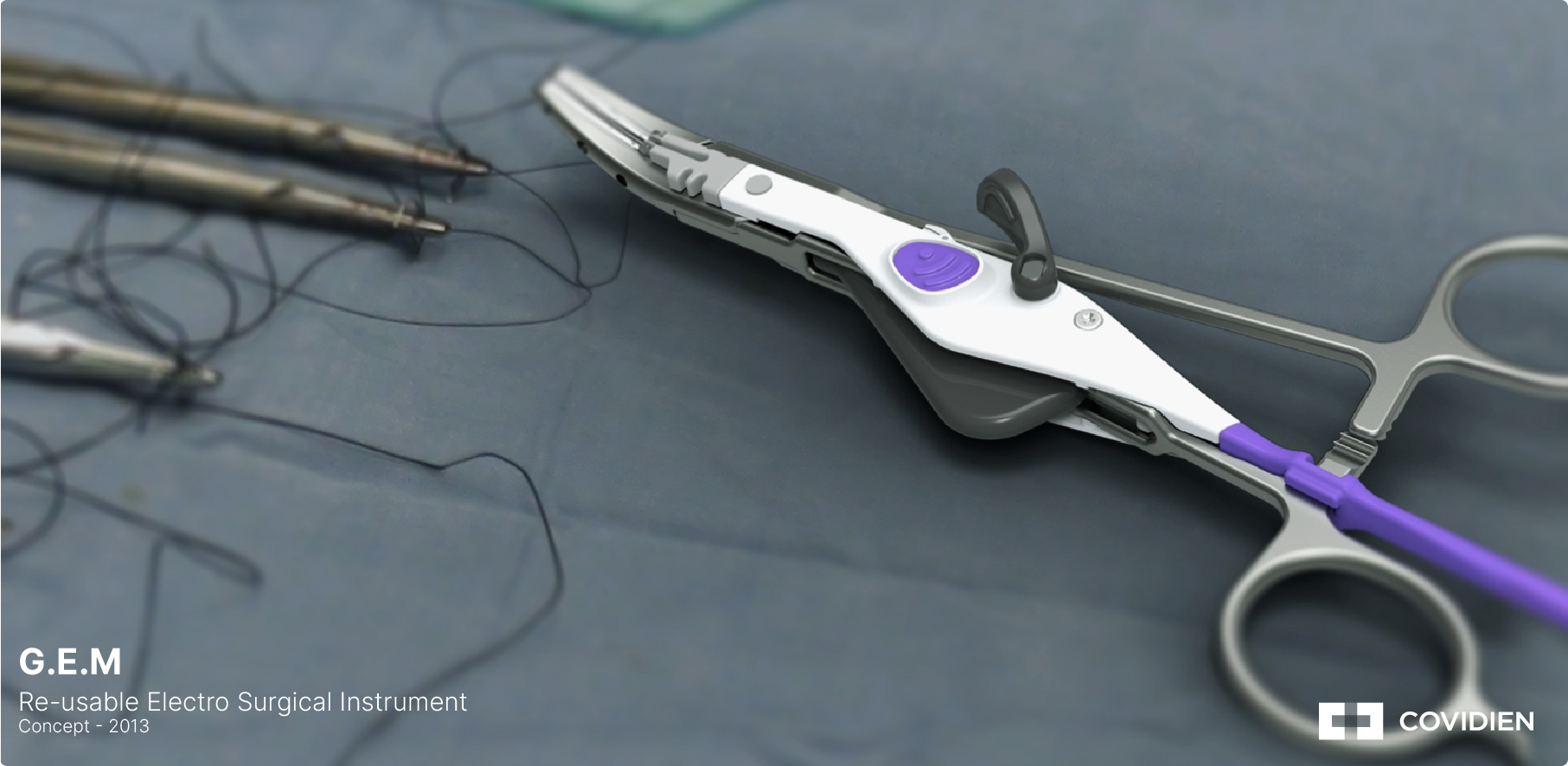
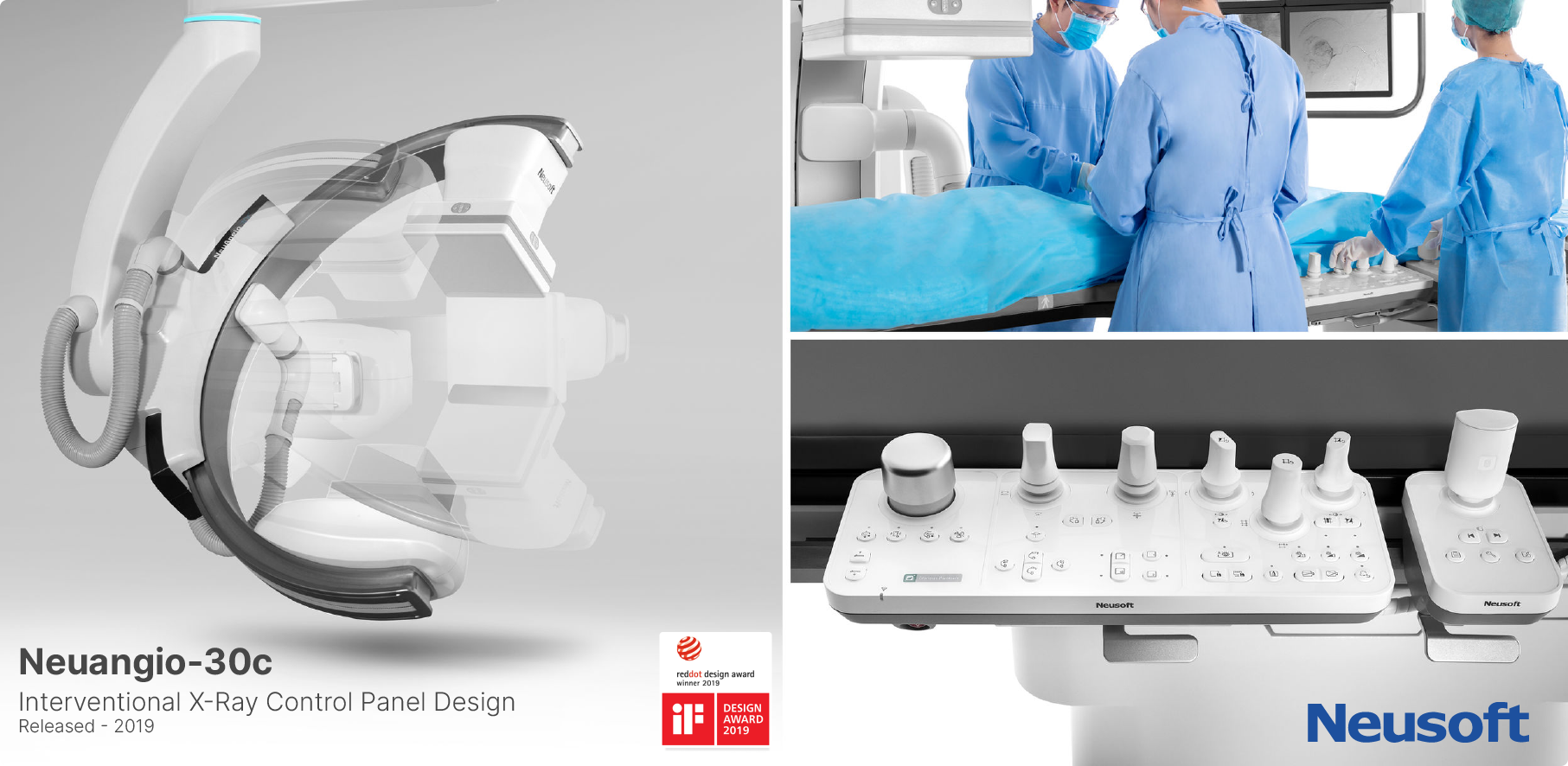
Key Customers
Key Customers
Process Overview
I am unfortunately unable to showcase indepth insights on this portfolion, I showcase here some of the key steps of the process I used with my team to support our customers goal. We could discuss these more indepth in the event of a direct call.
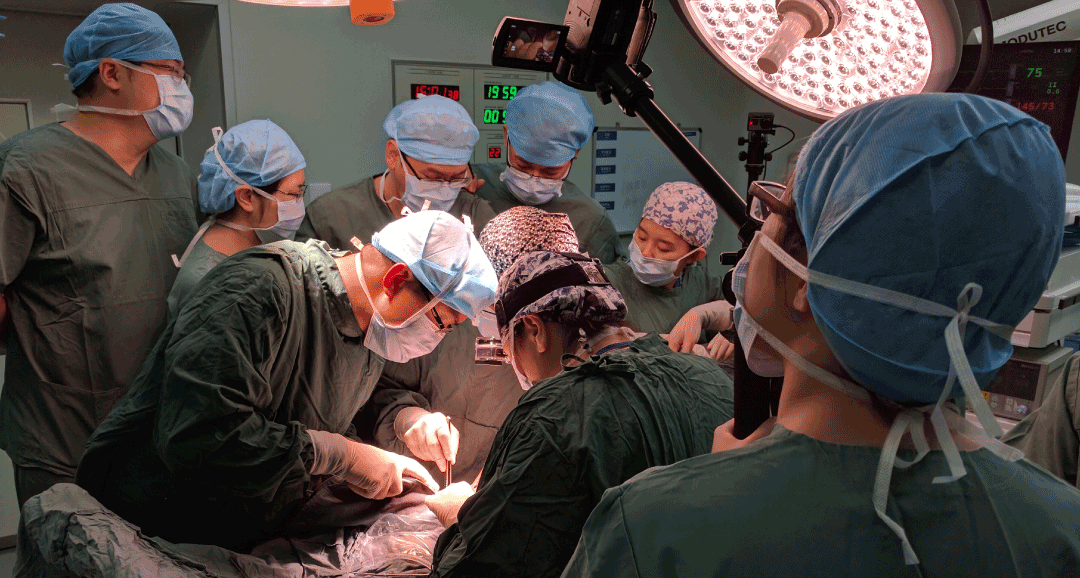
Field Research in Clinical and Non-Clinical Environment
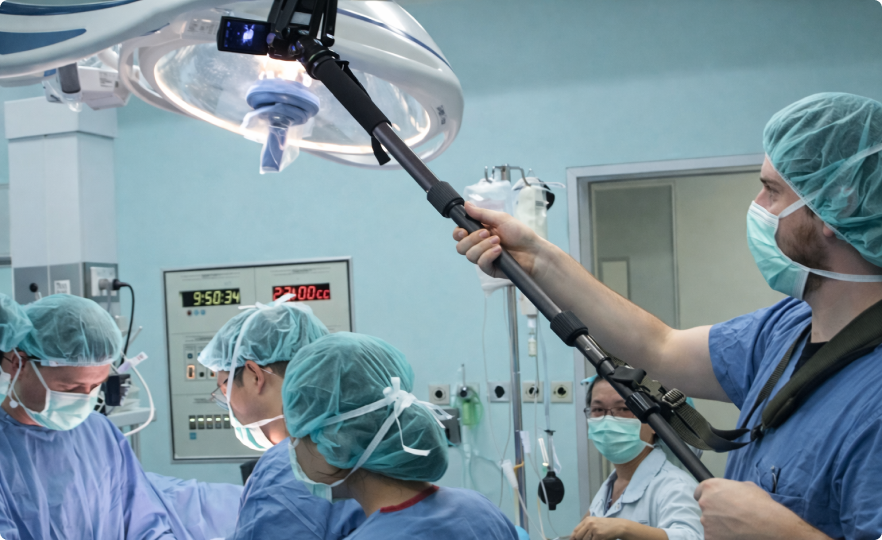
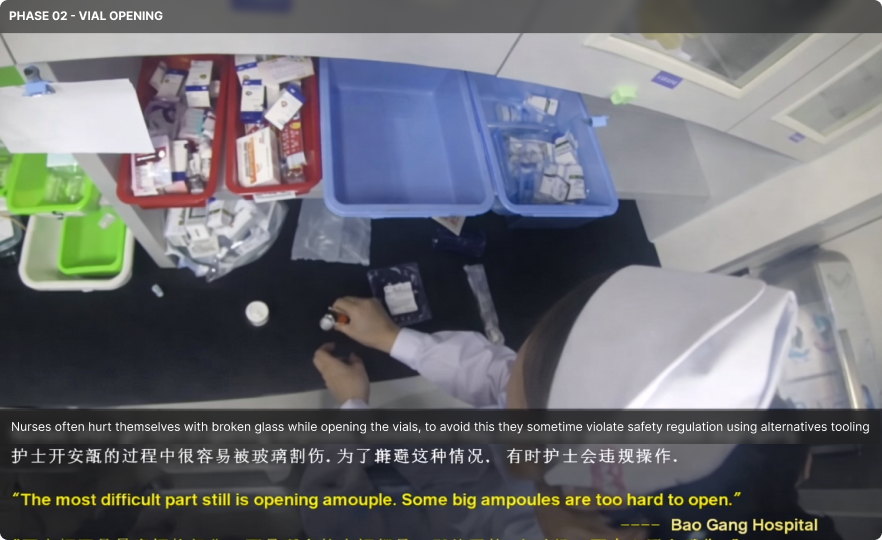
Field research informed every project and design decision. I worked closely with UX researchers in hospitals, operating rooms, and emergency departments to observe real-world clinical practices. This included documenting surgeries, building timelines, and comparing multiple cases to identify recurring patterns and opportunity areas. I also contributed to patient-focused research, mapping journeys to uncover constraints, unmet needs, and system-level insights across the care continuum.
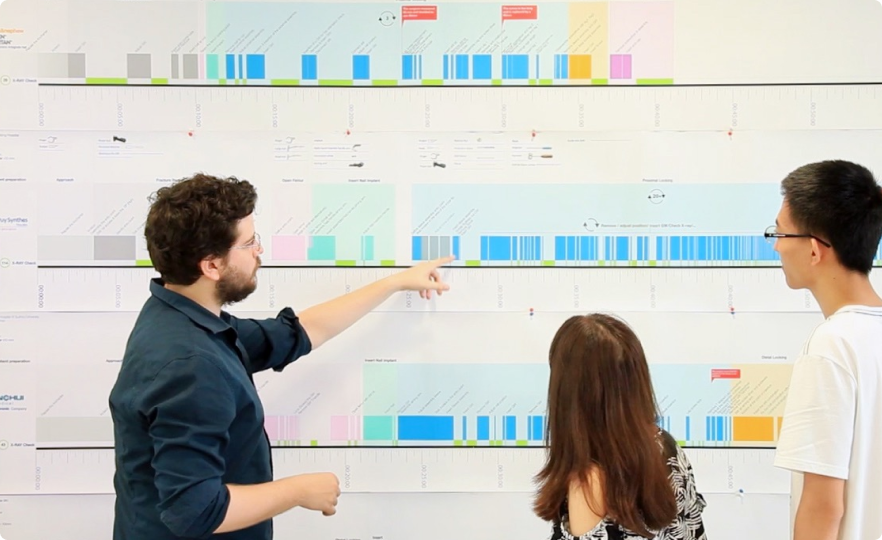
Co-creation & Alignement workshops
Co-creation workshops were a key milestone to share field insights with clients and bring practitioners and patients into the conversation. These sessions helped translate real-world experience into a shared understanding of needs and constraints. I designed and led multi-day workshops, defining their structure, facilitation, and outputs, with extensive preparation and synthesis work. Beyond ideation, these workshops built internal alignment across stakeholders and acted as a clear gateway into the next phases of product and service definition.
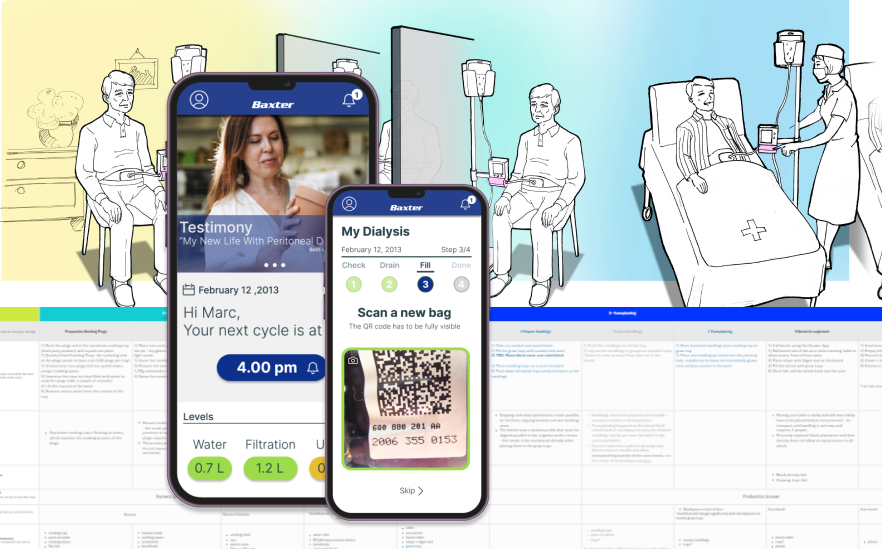
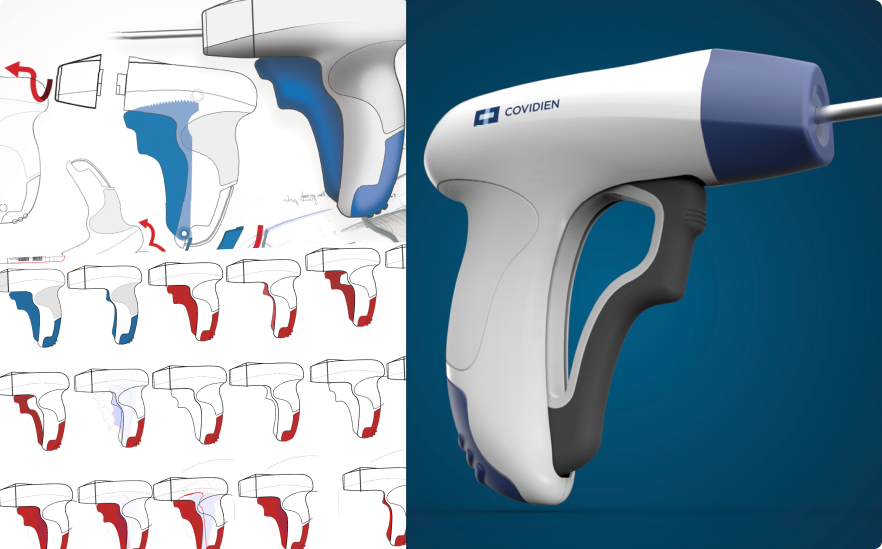
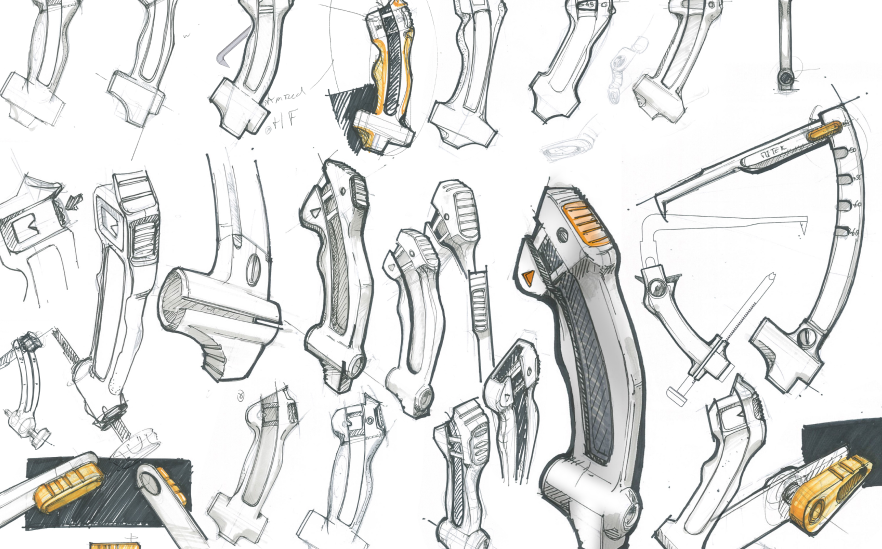
Concept Visualisation & development
I led industrial design and UX teams in early-stage ideation, concept framing, and visualization across hardware and software. This phase focused on translating research insights into clear design directions using sketches, 3D CAD, interaction flows, and system concepts. Projects often started as open, exploratory engagements and progressed toward defined product or service visions. Visualization was used as a tool for alignment and decision-making with client leadership, product and engineering teams, ensuring feasibility and shared understanding before deeper validation and prototyping phases.
IEC:62366 Compliant Formative & summative usability testing
I was trained as a human factors engineer to design and run IEC 62366 compliant usability testing, under the guidance of Serge Dubault VP Human Factor engineering at Worrel. This dual role as designer and human factors practitioner allowed me to integrate the right testing framework early in the design process. This approach reduced development time and increased the likelihood of successful regulatory validation with the FDA/CFDA by demonstrating early and continuous usability risk mitigation.
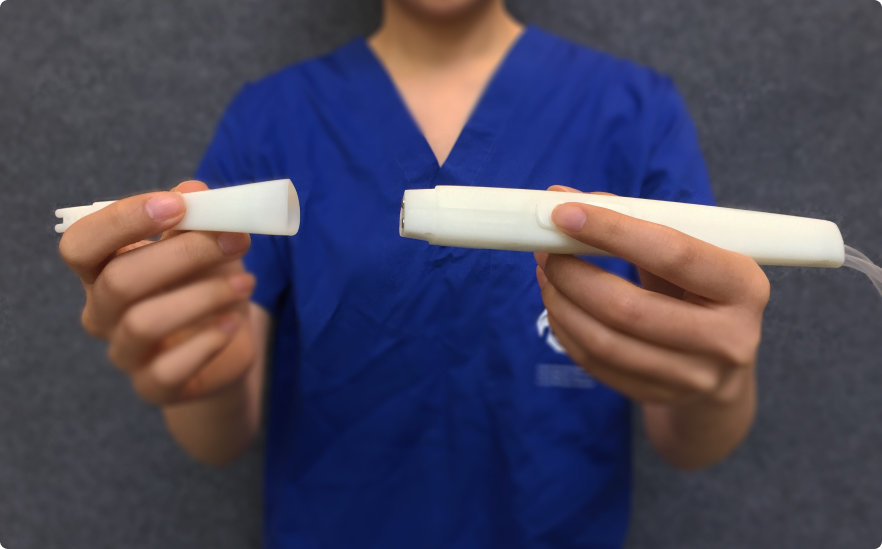
Low to High-Fidelity Rapid Iterative Prototyping
I led rapid prototyping efforts across industrial design and UI, defining testing strategies to validate concepts with users as early as possible. Prototypes ranged from low-fidelity paper and cardboard models to 3D-printed parts, interactive software wireframe, and hardware interfaces. While complex mechanical design was supported by design engineers, I guided teams on what to prototype, at which fidelity, and when to test. The focus was on iteration speed and user validation, supporting efficient progression from formative to summative usability testing.
Service and Operational workflow live pilots
For several projects, we ran live pilots in real hospital environments to validate solutions at system level. This included redesigning and deploying full workflows, from in-hospital IV admixture facilities to large-scale container-based labs. I supported clients through implementation, providing the necessary tools, concepts, and hardware to operate these pilots. Together with client product managers, we defined and monitored KPIs such as speed, cost, and operational efficiency. These pilots helped validate usability, integration within hospital systems, and real-world feasibility before full-scale rollout.